
SCN Lewis Structure (Thiocyanate Ion) YouTube
Using Lewis Dot Symbols to Describe Covalent Bonding This sharing of electrons allowing atoms to "stick" together is the basis of covalent bonding. There is some intermediate distant, generally a bit longer than 0.1 nm, or if you prefer 100 pm, at which the attractive forces significantly outweigh the repulsive forces and a bond will be formed.

Lewis dot structures SCN. Formal charges YouTube
Following steps are required to draw SCN - lewis structure and they are explained in detail in this tutorial. Find total number of electrons of the valance shells of sulfur, nitrogen and carbon atoms and including charge of the -1 charge Total electrons pairs in valence shells Determine center atom from carbon, nitrogen and sulfur atoms

Lewis Dot Structure for elements YouTube
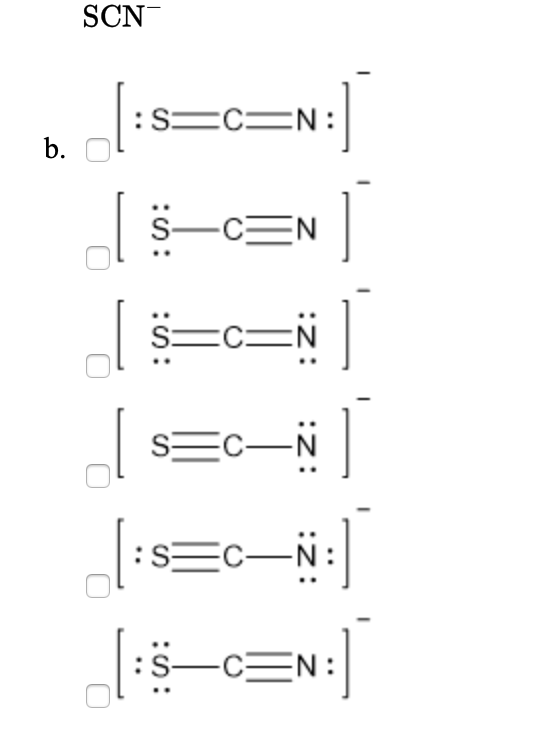
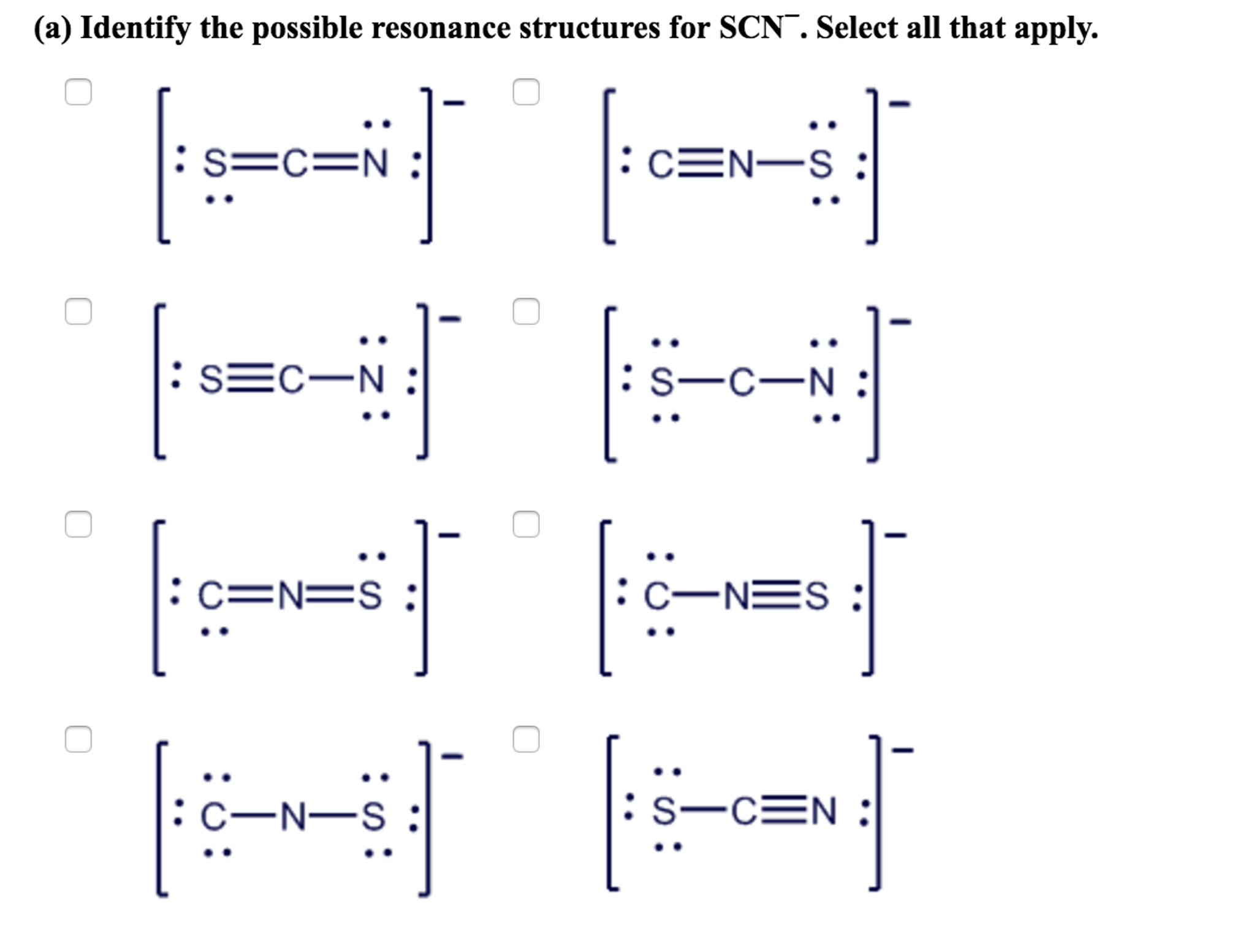
Draw a Lewis dot structure for [SCN]-. Is it one of the ones below? All of these molecules look different, but they fulfill the simple rules of Lewis dot structures. They all fulfill the octet rule and use 16 electrons (15 valence electrons +1 from the -). This demonstrates that in certain cases, molecules can have the same elements but it can.
Scn Lewis Structure
Using Lewis Dot Symbols to Describe Covalent Bonding. The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties.. Use the step-by-step procedure to write two plausible Lewis electron structures for SCN.

(Solved) RbIO2 Draw the Lewis dot structure for RbIO2. Include all
6 Steps to Draw the Lewis Structure of SCN- Step #1: Calculate the total number of valence electrons Here, the given ion is SCN- (thiocyanate ion). In order to draw the lewis structure of SCN, first of all you have to find the total number of valence electrons present in the SCN- ion.
Scn Lewis Structure
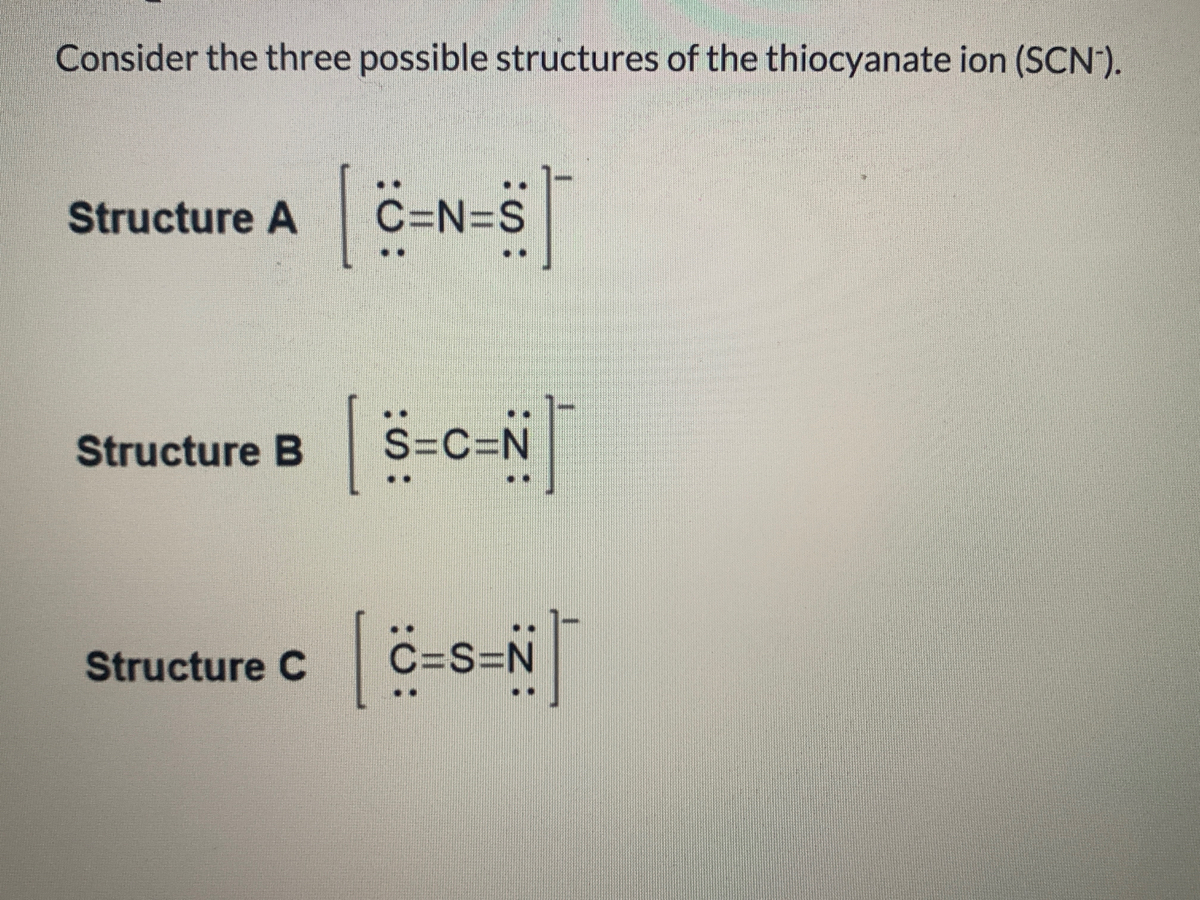
Step 1: Connect the atoms with single bonds. Step 2: Calculate the # of electrons in π bonds (multiple bonds) using formula (1): Where n in this case is 3. Where V = (6 + 4 + 5) - (-1) = 16 , V is the number of valence electrons of the molecule. Therefore, P = 6n + 2 - V = 6 * 3 + 2 - 16 = 4 So, there are : 2 double bonds or a triple bond.

Lewis Structure SCN plus dipoles, shape, angles, resonance and formal
0:00 / 3:24 • Intro SCN- Lewis Structure - How to Draw the Lewis Structure for SCN- (Thiocyanate Ion) Wayne Breslyn 725K subscribers Join Subscribe Subscribed 227K views 10 years ago A.
Identify the possible resonance structures for SCN^.
This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

SCN Lewis Structure How to Draw the Lewis Structure for SCN
SCN- Lewis Structure|| Lewis Dot Structure for SCN- ||Thiocyanate ion Lewis Structure#SCN-LewisStructure#LewisStructureforSCN-This video has solved the follo.

Scn Lewis Structure
The Lewis structure for SCN- has 16 valence electrons. SCN- Lewis Structure - How to Draw the Lewis Structure for SCN- (Thiocyanate Ion) Watch on See the Big List of Lewis Structures Transcript: This is Dr. B. We're going to look at the SCN- Lewis structure.

SCN Molecular Geometry / Shape and Bond Angles YouTube
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of.
SOLVEDThe thiocyanate ion, \mathrm{SCN}^{}, can form bonds to metals
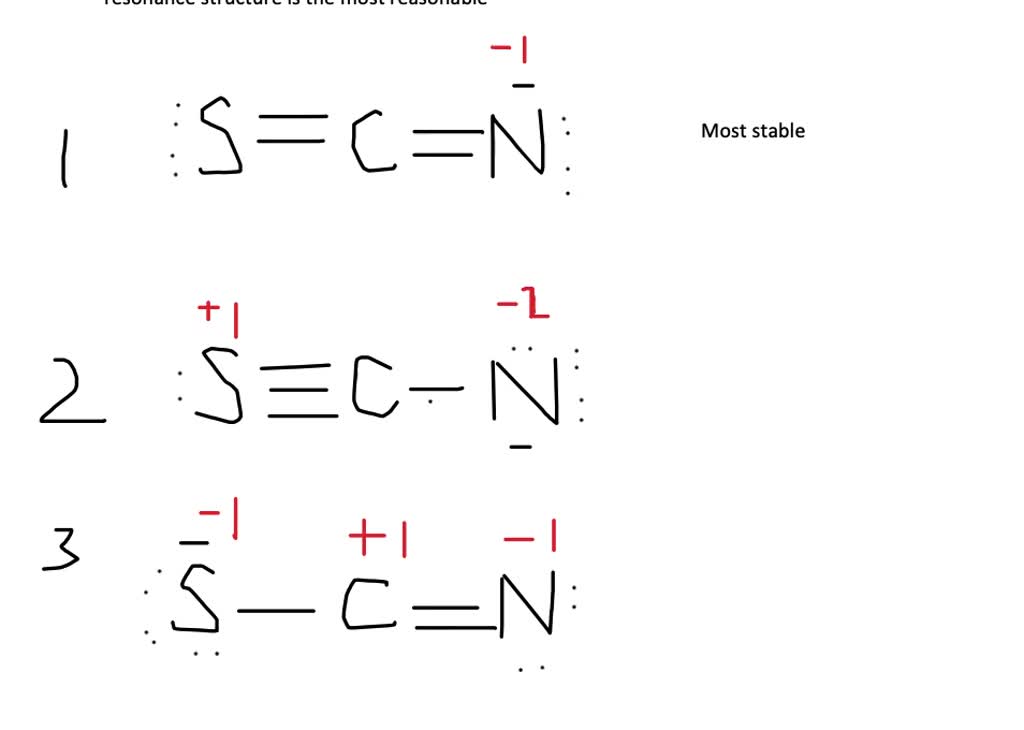
Use the step-by-step procedure to write two plausible Lewis electron structures for SCN −. Calculate the formal charge on each atom using Equation \(\ref{9.6.1}\). Predict which structure is the major contributor based on the formal charge on each atom and its electronegativity relative to the other atoms present.
How to Draw Lewis Dot Structure Grade 11 Chemistry YouTube
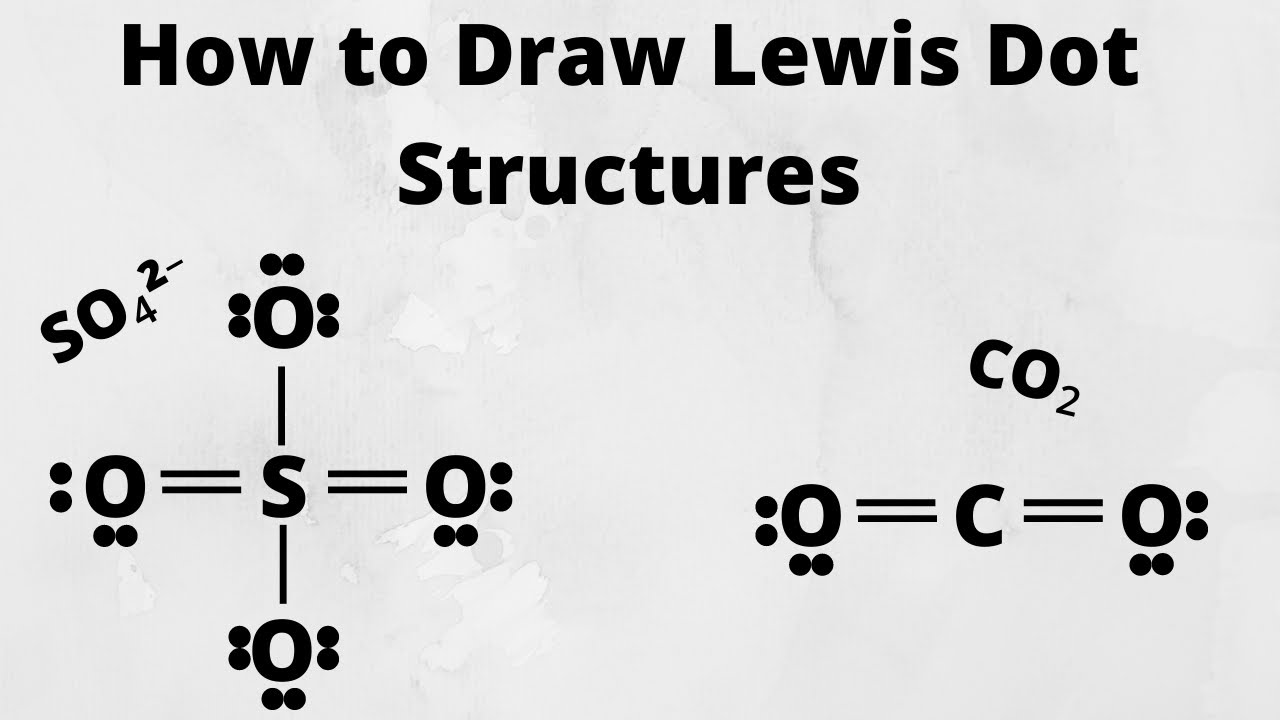
A Lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. When constructing a Lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose, or share.

RCSB PDB SCN Ligand Summary Page
2. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [ (2) (1) + 4 + 6] = 12 valence electrons. 3. Placing a bonding pair of electrons between each pair of bonded atoms gives the following: Six electrons are used, and 6 are left over.

SCN Lewis StructureHow do you draw the Lewis structure for SCN
Lewis dot structures: SCN-. Formal charges Diego Troya 5.5K subscribers Subscribe 160 12K views 5 years ago 13-8 This video explains the calculation and use of formal charges by drawing the.
SCN Lewis Structure, Molecular Geometry, Hybridization and Shape
The ion is made up of three atoms: Sulphur, Carbon and Nitrogen. SCN- Lewis Structure (Thiocyanate Ion) Watch on 0:00 / 3:31 The ion has a negative charge as it accepts one valence electron. In this blog post, we will look at the Lewis Structure, Molecular Geometry and shape of the molecule. Contents SCN- Valence electrons SCN- Lewis Structure